Curingenetics
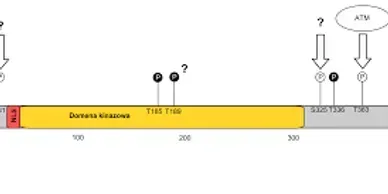
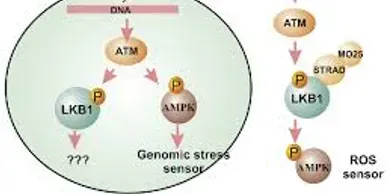
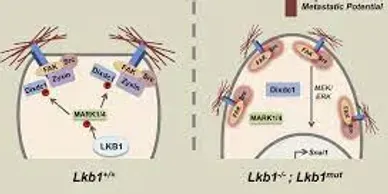
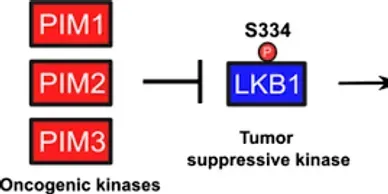
LKB1 (STK11) company
Repurposing FDA approved drugs for efficiency, speed, and cost effectiveness.
Diversified Scopes of Work (SOW) build on previous R&D efforts for identification of new candidate therapies to quickly commence clinical trials. Many agents approved for other uses have already been tested in humans, so detailed information is available on their pharmacology, formulation, and potential toxicity.
SOW1 (human phase)
Ruxolitinib, a Jak 1/2 inhibitor, significantly reduced tumorigenesis in a PJS mouse model. Based on the inventors' preclinical results, they expect Ruxolitinib to also reduce tumorigenesis in PJS patients. Other Jak inhibitors such as Itacitinib are also being engaged to reduce or stop polyposis and tumorigenesis.
SOW2 (human phase)
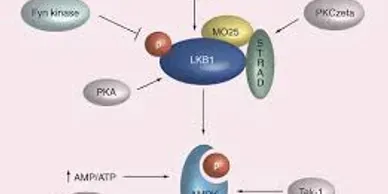
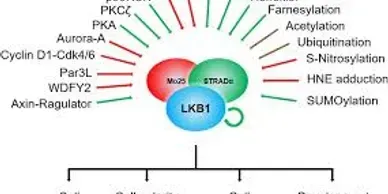
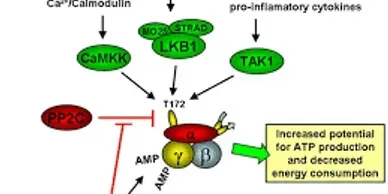
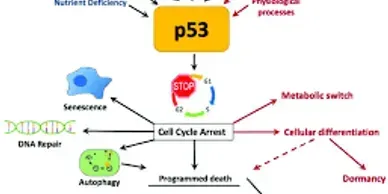
Rapamycin (Sirolimus), an mTOR inhibitor, is a promising candidate in treatment and prevention of PJS polyps. PJS is caused by mutation of LKB1 gene which leads to increased activity of mTOR pathway.
SOW3
Combo approach. Use the identified FDA approved compounds with Rapa and the mutation in cell lines and or animal models for treatment and repurposing.
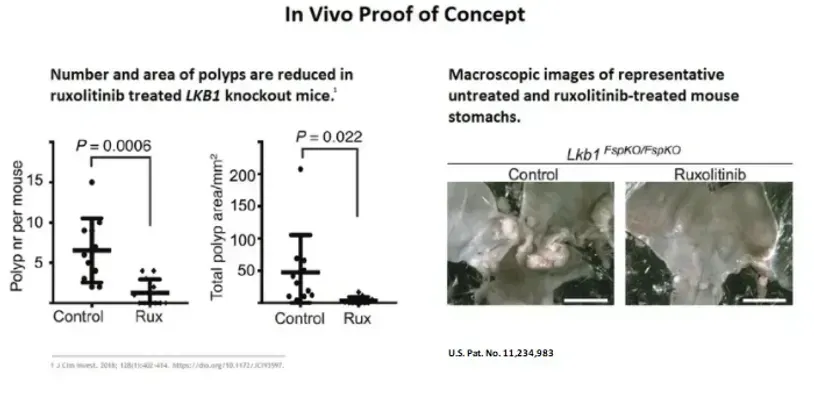
PJS Animal model proof
Polyps are reduced and eliminated in ruxolitinib treated LKB1 knockout mice.
SOW4
Utilize mRNA approach. Intestine delivery pill identified. Develop a vaccine to recognize the mutation and stop formation of polyps. Checkpoint immunotherapy may also be considered as a combo therapy.
SOW5
Stem cell therapy. Infuse corrected or donated stem cells to be proliferated as healthy T cells to supplement protein and correct deficiencies.
SOW6
Gene therapy and editing. Repair mutated LKB1/STK11 tumor suppressor gene.
SOW7
Liquid Biopsy. Lessen the burden of ongoing intensive screening and risks for patients.
Information on this site is not medical advice. Please consult with your doctor before making any medical decisions. By using our website you agree to cookies possibly tracking your activity. See terms of use and privacy policy. Curinggenetics is a 501(c)(3) non-profit public charity organization. Federal tax ID #86-2946082.
Copyright @ 2024 Curinggenetics